medical package validation test|packaging validation guidelines : dealer FDA considers ISO 11607 to be the paradigm validation protocol for medical device packaging. PROTOCOL DESIGN. The package validation protocol must be approached in stages, each of which becomes an issue when designing your package test systems. The first consideration involves the selection and qualification of the materials to be used in the . WEBUncover our selection of the top 10 online casinos for 2024, which includes a range of trustworthy and superior gaming sites. Each casino is carefully reviewed, ensuring .
{plog:ftitle_list}
Salmos 65:8. Bom dia! Deixe as suas preocupações de ontem para trás. Hoje é um novo dia para agradecer a Deus. Não se preocupe, pois Ele estará cuidando de você hoje. Tenha um dia abençoado pelos braços .
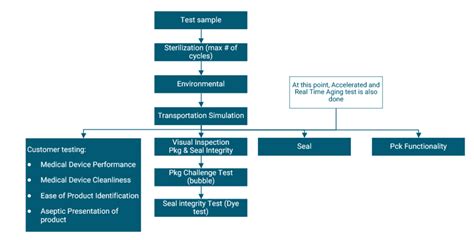
the standard series iso 11607 stipulates validation of the packaging processes used for industry, health care facilities and wherever medical devices are pack-aged and sterilized (examples of . Assurance that your package will provide an effective, consistent sterile barrier for your medical device requires a well-designed, thoroughly documented test protocol evaluating .Heat seal validation requires a series of tests to determine optimal sealing parameters for specific medical device packaging. These parameters can include temperature, pressure, and time. Validation also verifies that the sealing process will produce consistent seals able to meet strength, integrity and uniformity requirements, and can .Our specialized packaging validation services: Medical Device and Combination Products Package Testing Medical device and pharmaceutical manufacturers are required to obtain 510(K) approval on each package. DDL performs a variety of testing services to ensure the integrity of the medical device packaging and compliance with ISO standards.
FDA considers ISO 11607 to be the paradigm validation protocol for medical device packaging. PROTOCOL DESIGN. The package validation protocol must be approached in stages, each of which becomes an issue when designing your package test systems. The first consideration involves the selection and qualification of the materials to be used in the .Burst Test Whole package inflation tests are categorized as burst tests, creep tests or creep-to-failure tests (ASTM F1140 or F2054). To perform a burst test, a package is inflated at a uniform rate until the seal separates at the point of greatest weakness. The burst test is a peak inflation pressure test. It is a variable test;
What goes behind the seal of every medical device package is the rigorous work of laboratories. According to ISO 11607-1 and ISO 11607-2, laboratories should use validated test methods to ensure that the packaging complies with international standards. These tests mimic the conditions a package might face, from storage to transportation, and . Updates to ISO 11607, Parts 1 and 2, have left many medical device manufacturers wondering about the future of their packaging designs. . Package Testing and Validation 101. . Package integrity and seal strength limitations must be evaluated before packaging is ready for initial validation. OEMs can test the physical properties of their .The package validation protocol must be approached in stages, each of which becomes an issue when designing your package test systems. The first consideration involves the selection and . F1585 Medical Packages ASTM Standard Test Method for Detecting Seal Leaks in .be available for validation. this process comprises: 4.1 drafting of a validation plan 4.2 Validation of packaging processes 4.2.1 installation qualification (iQ) 4.2.2 operational qualification (oQ) 4.2.3 Performance qualification (PQ) 4.3 drafting of a validation report 4.4 Formal approval of validation 4.5 Process control and monitoring
Some labs report up to 30% of their medical device packages fail the ASTM or ISTA transit tests. • It’s important to conduct a sterile presentation test with end-users. The test ensures the device can be aseptically presented. Some packages allow the device to gently fall onto a sterile table while others require manual removal.Packaging Compliance Labs (PCL) is an ISO 17025-accredited lab that offers medical device packaging validation, packaging engineering, and ISO 13485-certified contract packaging.Package testing helps evaluate the packaging’s ability to maintain the device’s properties over time, allowing manufacturers to determine appropriate expiration dates. Expert ISO 11607 Packaging Validation Testing of Medical Devices. Keystone Package Testing provides expert medical device package testing.ISO 11607-2:2019 — Packaging for terminally sterilized medical devices — Part 2: Validation requirements for forming . or bad aseptic presentation. In fact, the primary package is a minimum package that prevents ingress of microorganisms and allows aseptic presentation at the point of use. . The established routine tests used during the .
For medical devices, package validation testing is outlined in ISO 11607. The test consists of seal integrity (seal strength), material integrity (bubble leak), distribution testing, and package aging. . Expert Medical Device Packaging Validation Test Lab. Companies partner with us for package testing because of our high-quality work. We take .
purpose of package testing

purpose of medical package testing
Updates to ISO 11607, Parts 1 and 2, have left many medical device manufacturers wondering about the future of their packaging designs. These changes come at a stressful time for OEMs, as Europe is also in the process of replacing the current Medical Device Directive (MDD) with the Medical Device Regulation (MDR) in 2020. The interplay between ISO 11607 and MDR is .Medical device validation is an arduous and challenging process, there is no need to go through the process alone and unsupported. . Medical package testing takes place in our ISO 17025-accredited, state-of-the-art facility. From .

We test and validate packaging for medical device design history files and regulatory submissions, and we support continued testing for your validated device. +1 (888) 794-0077. . package validation includes accelerated aging and the corresponding integrity testing as well as simulated distribution and its subsequent integrity testing.
ISTA Test Methods – (includes all procedures and projects) Package Integrity and Seal Strength Tests. Advance Packaging Technology Laboratories conducts package integrity and seal strength tests to check the packages its ability to .• Want to test if the MEAN is greater than 90. –The 95% Lower Bound on 95.1 is 94.8. This implies if we sample 20 units from a population whose mean is 95.7 with SD=2.4, the average will be greater than 94.8; 95% of the time. • Want to test if the INDIVIDUAL values are greater than 95. –The Lower Bound with 95% confidence and 99% coverage– a material used in medical packaging which is intended . in validation results. • Some test methods are actually processes that prepare materials for evaluation by test methods. Test Method Key Points. . Whole package test. Porous or .
As a leader in dye penetration package testing, Keystone Compliance understands the importance of detecting seal leaks in porous medical package testing. Our knowledge of packages has allowed us to create a streamlined approach to medical device package validation. We walk customers through the process from test plan to test report.systems and/or materials used in conjunction with sterile medical devices. Beginning with a review of the importance of packaging validation for medical devices, the white paper then presents a summary of ISO 11607, the standard for packaging materials used for sterilized medical devices, and provides details on Therefore, the tests we perform in this phase are slightly different than the integrity tests we perform when we are in IQ/OQ/PQ (ie: dye leak testing). Bubble Leak Test is a great test for testing the whole package because we are submerging the entire package underwater. Once the package is submerged and we have increased the internal pressure .
A pressure decay test which is very precise, it requires tooling. Calibrated hole test. In this test impermeable packs are fitted with laser drilled holes of 12.5, 25, or 50 micron diameter. Leak from the packs is then measured using a vacuum decay method and compared to non-perforated packs. MET uses this test for validationMedical device packaging transit testing is a critical part of your sterile medical device packaging validation. It is required by ISO 11607-1, the recognized guidelines for terminally sterilized medical devices. This conditioning cycle proves that your product will arrive at the hospital or medical facility, sterile and ready to go. Thanks Ronen and Pads38, I reached out to a friend outside the cove and summarized what I've learned: 1. It is required in the EU; See MDD, Annex I, section 8.6: "Packaging systems for non-sterile devices must keep the product without deterioration at the level of cleanliness stipulated and, if the devices are to be sterilized prior to use, minimize the .
Assessing and executing test protocols for package design, packaging processes validation, and package shelf life testing are how MedPack Labs, LLC, keeps your medical device package compliant with ISO and ASTM standards. Take advantage of our sterile barrier package testing services for an easier 510(k) application or a smoother PMA approval.The Dye Penetration Test Kit or Dye Leak Test is an observational technique used to detect possible imperfections in the closure of a sterile barrier system, mainly employed for medical equipment. This examination provides important data concerning any material or packaging process flaws and is specifically important for validating the seal durability of medical devices. .

packaging validation guidelines
working principle of rockwell hardness tester
Resultado da 19 de dez. de 2022 · Gaby Fatal ⋆ Travesti Com Local. Piraju, SP, Brasil. Piraju ›. Piraju, SP, Brasil. ver no mapa. Anunciante desde 19/12/2022. Documentos verificados . (14) 99610-5764. Me chama no Whatsapp CLIQUE AQUI. Apenas contato profissional ou será bloqueado. Informe que a viu .
medical package validation test|packaging validation guidelines